The news is in: Prof. Koen Binnemans (SOLVOMET KU Leuven) has just been awarded a second ERC Advanced Grant for his groundbreaking project on developing a solid basis for circular hydrometallurgy (“CIRMET”). SIM² Director and SOLVOMET Innovation Manager Peter Tom Jones sat down with Koen in order to better understand why this second ERC Advanced Grant is so unbelievably important for the man who has been an opinion leader in the field of sustainable metallurgy for so many years now. In this open-hearted interview you will learn more about Koen’s circular hydrometallurgical thinking and why he has given up on the use of ionic liquids or deep-eutectic solvents in the field of extractive metallurgy.
Interview highlights
“Genuine breakthroughs in hydrometallurgy will not come from the use of neoteric solvents like ionic liquids or deep-eutectic solvents, but rather from a deep understanding of hydro-processes at a molecular level. Hydrometallurgy needs to evolve to low-energy-input circular hydrometallurgy.”
“CIRMET targets to make hydrometallurgical flowsheets circular by using well-known chemicals, by better understanding at a molecular level of the chemical reactions involved, and by clever manipulation of chemical equilibria.”
“The concept of solvometallurgy remains useful but only in very specific cases where there is an added value, such as in the purification of lithium salts.”
“The use of ionic liquids (ILs) and deep-eutectic solvents (DES) in extractive metallurgy is, in my view, a dead-end street.”
“In my research philosophy, there is a constant movement from low to high-TRL research, and back from high to low TRL. This is circularity at a meta level.”
First of all, my very sincere congratulations for this remarkable achievement. Your second ERC Advanced Grant. What is the overriding feeling?
Sometimes, I am still asking myself whether I am dreaming. I find it difficult to believe I was able to win a second ERC Advanced Grant. Believe me, this achievement was not a walk in the park. For my first ERC Advanced Grant, SOLCRIMET, I was successful the first time I submitted my project proposal. For CIRMET, I had to try three times before I finally succeeded. The competition is fierce.
Moreover, for the past 2 years, in the second round of the evaluation process, the ERC organised interviews for the candidates in the latter stages of their applications for ERC Advanced Grants. I can assure you that preparing for such an interview is very stressful. Don’t forget that the expert panel members have read the referees’ comments, whereas the ERC candidate doesn’t have any idea about their content. So, it is very difficult to prepare yourself.
Anyway, it’s done now. More and more, my original feeling of disbelief has transformed into a feeling of being very proud. You cannot ignore that the ERC Advanced Grant is one of the most prestigious grants a scientist can get. And this is my second one. So, yes, I am thrilled.

Your first ERC Advanced Grant SOLCRIMET was all about the development of “solvometallurgy”, as a radical alternative for hydrometallurgy. Some kind of clean slate. In your new project, CIRMET, you want to develop the concept of “circular hydrometallurgy”. So rather than promoting a completely new alternative for hydrometallurgy you are promoting a modified version. From revolution to evolution. Why this change of heart?
Look, I still find the concept of solvometallurgy very fascinating, because in organic solvents you can carry out chemical reactions that are impossible or much less selective in aqueous solutions.
The coordination chemistry in polar organic solvents is often very different from that in water because of differences in the solvation of metal ions. By leaching ores or concentrates in organic solvents you can avoid problems with gel formation. Separation factors for metal ions in non-aqueous solvent extraction can be very different from what is observed in conventional solvent extraction. You can carry out solvent extraction with water-sensitive metal compounds. In non-aqueous electrolytes, you can electrodeposit reactive metals that cannot be obtained from aqueous electrolytes. Examples include aluminium and the rare earths.
Binnemans, K., Jones, P.T. Solvometallurgy: An Emerging Branch of Extractive Metallurgy. J. Sustain. Metall. 3, 570–600 (2017). https://link.springer.com/article/10.1007/s40831-017-0128-2
That being said, during the development of our solvometallurgical processes we have been confronted with unexpected issues involving the instability of the organic solvents. Not only in SOLVOMET, but also in other EU and national projects. For instance, in the EU project TARANTULA we have developed a solvometallurgical process for the recovery of tungsten from scheelite (CaWO4); the process comprised leaching of the scheelite concentrate with hydrochloric acid dissolved in ethylene glycol. The process worked much better than in water since we had no gel formation of tungstic acid, and we were very enthusiastic about it… until we noticed that HCl reacts with ethylene glycol, resulting in the formation of small amounts of the very toxic compound 2-chloroethanol. Also, in the case of electrodeposition in non-aqueous electrolytes, we have been facing issues with decomposition of the solvent. Non-aqueous solvent extraction suffers from the large mutual solubility of the two “immiscible” organic solvents.
However, I still see several opportunities for solvometallurgy in the field of antisolvent crystallisation and especially for the purification of lithium salts, as explored in my Proof-of-Concept grant SOLVOLi. So to reiterate, in some cases solvometallurgy makes senses; in others it doesn’t.
Can you summarise what CIRMET is all about?
To use the on-liner of Anders Sand (Boliden) in your documentary on responsible mining in Europe, “without metals there is no green transition”. Indeed, large amounts of high-purity metals, including cobalt, nickel, manganese, lithium and copper, are required to fuel the transition to a climate-neutral economy. However, producing them is not that straightforward.
It is a paradox that the metals needed for clean energy and clean mobility are produced by “not-always-so-clean” hydrometallurgical processes, which consume large amounts of chemicals and generate massive volumes of waste. To solve this paradox we need to shift from the present linear, waste-generating hydrometallurgy to low-energy-input circular hydrometallurgy.
Binnemans, K., Jones, P.T. The Twelve Principles of Circular Hydrometallurgy. J. Sustain. Metall. 9, 1–25 (2023). https://link.springer.com/article/10.1007/s40831-022-00636-3
Circular hydrometallurgy refers to the design of energy- and resource-efficient flowsheets that consume minimum quantities of reagents and result in minimum waste. This is what CIRMET is all about. We can make flowsheets circular by using well-known chemicals, in combination with a better understanding at a molecular level of the chemical reactions involved, and by clever manipulation of chemical equilibria.
By the ingenious use of solvent extraction in ways never attempted before, in CIRMET we will develop novel approaches for circular, low-energy hydrometallurgical flowsheets that consume no chemicals other than hydrogen and carbon dioxide. In CIRMET, there are 3 levels of innovation: at the molecular level, the unit process level and the flowsheet level. It seems the reviewers liked this approach.
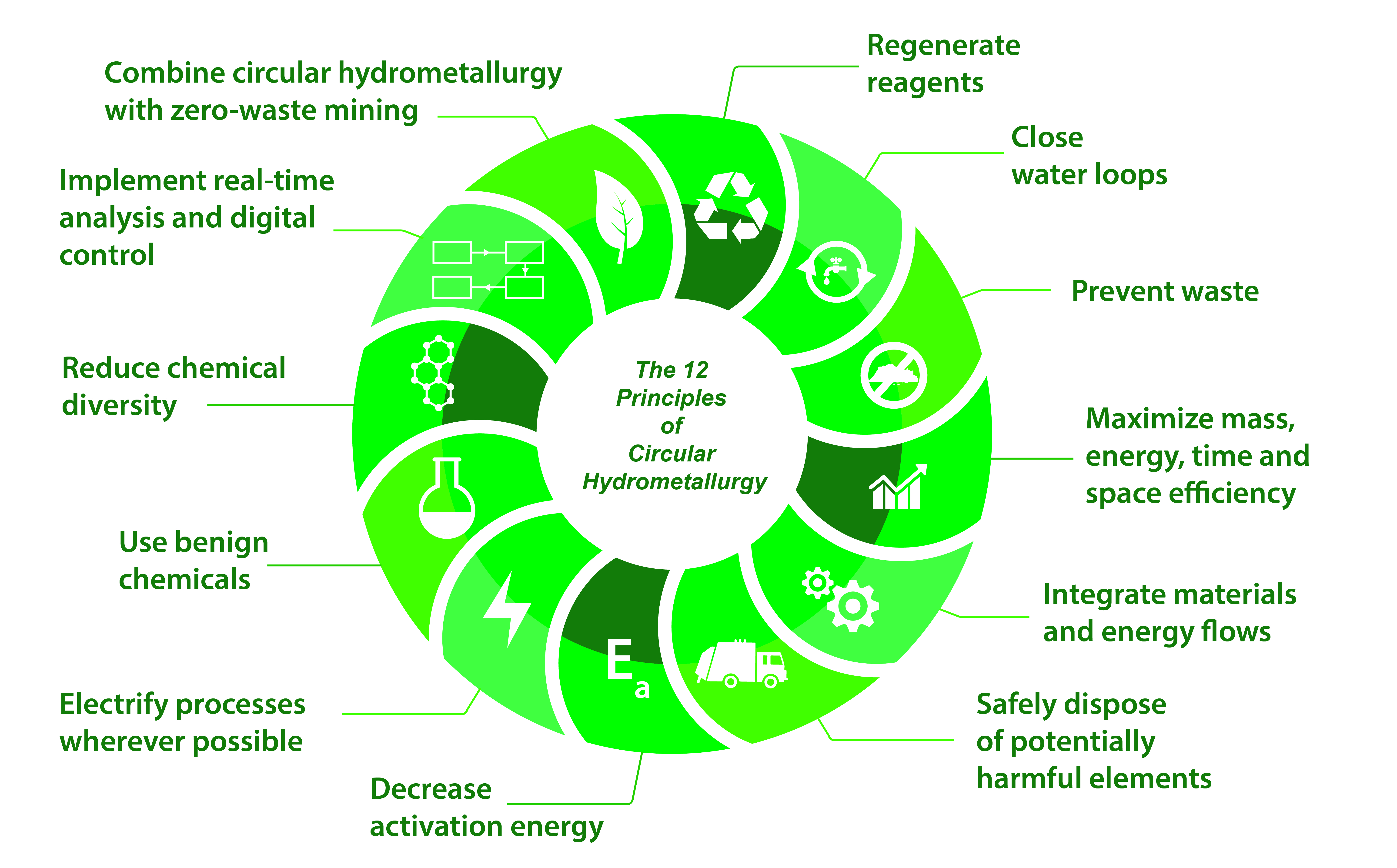
Figure 1. The Twelve Principles of Sustainable Metallurgy, as published in the Journal of Sustainable Metallurgy
In the past you have spent a lot of effort in the promotion of ionic liquids and deep-eutectic solvents as alternative solvents for metallurgy? You have published about 200 papers on the topic. However, in CIRMET you state that breakthroughs in hydrometallurgy will not come from the use of those “neoteric solvents” but rather from a molecular understanding of the processes? So does this mean you have given up on ILs/DES?
This might come as a shock to some but, absolutely, I have given up completely on these solvents. Although I am still convinced that “solvometallurgy” (using organic solvents in extractive metallurgy) holds some promise, I am much more pessimistic about the use of ionic liquids (ILs) and deep-eutectic solvents (DES) in metallurgy. For me, this is a dead-end street and we should be honest about this.
Based on my research experiences the past 10 years, I have come to the conclusion that there are too many disadvantages connected to these solvents. Soon, a new “opinion paper” of ours – "Ionic Liquids and Deep-Eutectic Solvents in Extractive Metallurgy: Mismatch between Academic Research and Industrial Applicability" – will be published in the Journal of Sustainable Metallurgy in which these disadvantages are discussed in detail.
They include: (1) issues with high viscosity; (2) limited chemical stability under the conditions of metallurgical processes; (3) difficulties with recycling and reuse; (4) a lack of demonstrated unit processes and flowsheets on the pilot scale; (5) insufficient material-property data available for engineering purposes; (6) the administrative burden of obtaining licenses and safety permits; (7) very high costs for large-scale operations; and (8) minimal added value compared to state-of-the-art hydrometallurgical processes.
Binnemans, K., Jones, P.T. Ionic Liquids and Deep-Eutectic Solvents in Extractive Metallurgy: Mismatch between Academic Research and Industrial Applicability. J. Sustain. Metall. In press.
I want to stress that I am not making a statement about the applicability of ionic liquids and deep-eutectic solvents in general, but specifically about their applicability in extractive metallurgy. For the latter, as I said before, I feel like this is a dead end. We should change course and pursue more realistic avenues. I believe that’s a very important message.

Yes indeed. Not sure how your international colleagues will receive that message but, I’m all in favour of directing research into realistic approaches, rather than trying to “push the sky away”. So let’s come back to your new CIRMET project. CIRMET proposes to develop a predictive thermodynamic framework for hydrometallurgical unit operations like the solvent extraction of metals or acids. How realistic is this? What are the challenges?
Indeed, central to CIRMET is the predictive thermodynamic modelling of solvent-extraction equilibria. This is a far from a trivial task. We are dealing here with the most difficult kind of equilibria to treat with computational methods: heterogeneous equilibria involving two immiscible liquid phases with varying ionic strengths and varying chemical compositions.
For nearly 150 years, since the work of the Josiah Willard Gibbs and Walther Nernst, scientists have been struggling with this challenge. By using chemical models and not physical ones, the mathematical expressions describing the thermodynamics are less complex. Also very important in CIRMET is the use of Gibbs energy minimisation (GEM) to determine the equilibrium composition of the aqueous and organic phases in contact with other. It will remain a challenge to identify which species are present in solution, as well as their standard and excess thermodynamic properties. For this reason, advanced microcalorimetric measurements will be used in CIRMET.
In terms of the metals you address in CIRMET, you will focus on the battery raw materials, and more particular on Ni, Co and Mn. In the past, most of your papers have dealt with the extraction and refining of rare-earth elements (REEs) ? Any specific reason why you have gradually changed course?
In SOLVOMET we are still working on rare-earth elements. For instance, in the Horizon Europe project EXCEED. However, although SOLVOMET’s activities have focused on rare-earths projects, and even more specifically on the recycling of rare earths from magnets, our activities are now much more diverse than just research on rare earths. We are developing process for a diversity of “clusters of metals” ((1) battery metals (lithium, cobalt, nickel, manganese); precious metals (PGMs, gold, silver), refractory metals (tantalum, niobium and tungsten)). Furthermore, there has also been a gradual shift where recycling is complemented by refining primary materials.
There are several reasons for these changes, one of them is that we do not have rare-earth mining in Europe yet, in contrast to the mining of battery metals. Additionally, there is a practical reason. It is not easy to demonstrate the separation of rare earths by solvent extraction in a laboratory, because you need a very large number of mixer-settlers or other contactors. It is also not convenient to work with hundreds of litres of feed solution and large volumes of organic solvents in a university lab. For the separation of cobalt from nickel, this is completely different, and thus more practical.
But to be clear: I am still very much interested in the metallurgy of rare earths. Don’t forget that I have 30 years of experience in rare-earth research. I do not want that this expertise to be lost. So, I’m looking forward to new projects on the chemical recycling of magnets from end-of-life electric vehicles.
With this new project you will be returning to rather basic, low-TRL research? How will you combine that with the on-going development to further grow your SOLVOMET Industrial Service Centre? Is there a contradiction between low-TRL and high-TRL foci?
No, there no contradiction between low-TRL and high-TRL research whatsoever. Part of my success as a scientist is due to the cross-fertilisation between high-TRL and low-TRL projects. The general perception in innovation is that you gradually proceed from low TRL to high TRL, from an idea, over proof-of-principle in the lab, demonstration in mini-pilot scale, to full pilot scale and finally a commercial process or product. However, for me, the feedback loop is equally important. I like seeking for patterns everywhere, including for loops than can be closed, not only material loops but conceptual loops as well..
In SOLVOMET, the bilateral projects with industry, which are high-TRL projects, very applied ones, generate ideas for fundamental research. In high-TRL projects you are being exposed to practical problems that sometimes need further investigation at a more fundamental level in order to overcome them. I don’t just want to solve problems; I want to understand the underlying molecular mechanism. To be brutally honest, I often get more original ideas from my interactions with colleagues in the metallurgical industry than by attending scientific conferences or reading scientific literature. It’s just a totally different type of exposure, which forces you to constantly challenge your own perceptions and ideas.
So, in my research, there is a constant movement from low- to high-TRL research and development, and back from high to low TRL. This is circularity at a meta level. This is also the reason why I strongly object to transforming SOLVOMET Industrial Service Centre into a spin-off company. Some of my colleagues are criticizing us for becoming more and more “a company within the university”. I do not agree with this. Without the bilateral projects with industry, I would never have been able to write the CIRMET project, which is all about basic science.

As an Industrial Research Manager at KU Leuven, I thoroughly agree with that conclusion. In my view research at a more fundamental level should be somehow in sync with more applied research. Your work at SOLVOMET is testament to that belief. On a different level, can you say something about how you are able to come up with new ideas for both fundamental and applied projects. Do you have a specific method for this that you are willing to share with “the world and its wife”?
Absolutely. I think that the most important one is what I call “focus time”: 7 days a week (weekends included), I block up to 2 hours of focus time in my agenda. During this time, the only activities I allow myself to do is thinking, reading and writing. This is time for deep work. No internet, no email, no distractions. Additionally, I try to walk for about 1 hour per day in the fields or forests. This puts my unconscious mind at work.
In fact, most of the ideas for the CIRMET project popped up in my mind during these walks, definitively not when I was working at my desk. Also worth mentioning is that I stopped watching television about 25 years ago. I read a lot of books. Mind you, no e-books. I want to hold and feel the book. I never read novels, but often biographies and history books. In the past few years, I also started reading books on philosophy. I really enjoy reading about ancient stoicism, such as the Meditations of Marcus Aurelius, the works of Epictetus and Seneca.
Not my cup of tea, but I see that it somehow inspires you. That’s key. To close off, you are 53 years old now. You obtained your 2nd ERC Advanced Grant. What drives you now? Which ambitions do you still have? Will Koen Binnemans be a happy man when he retires in 15 years or so.
First of all, I hope that I will still be in good health at the time of my retirement. After being seriously ill in 2019, I know how important good health is. And I want to stay scientifically active as long as possible. I am not a person who likes to be in a management position.
SOLCRIMET was my first one, CIRMET is the second one, and maybe I will try in 5 years to go for a third ERC AdG. But first, my focus will be on CIRMET and on the development and implementation of circular hydrometallurgy backed up and supported by my wonderful SOLVOMET team. I really want to make a difference towards a greener alternative to the incumbent hydrometallurgical flowsheets. That’s my contribution to the transition to a climate-neutral economy.
And a very welcome one. Thank you for this interview and I wish you the best of luck with what comes next. Looking forward to collaborating during the next 15 years!
[Interview: Peter Tom Jones] [Photos: Elisabeth De Decker]
Biography Koen Binnemans
 Koen Binnemans was born in Geel (Belgium) in 1970. He obtained his M.Sc. (1992) and Ph.D. (1996) degrees in Chemistry at the University of Leuven (KU Leuven). In the period 1999−2005, he was a postdoctoral fellow of the Research Foundation Flanders. He did postdoctoral work at the universities of Rennes (France) and Exeter (UK). From 2002 until 2005 he was (part-time) associate professor, from 2005 until 2010 professor, and since 2010 he is full professor of Chemistry at the University of Leuven. Koen is an inorganic chemist who is combining fundamental and applied research in a unique, double-sided interaction model. His main field of expertise could be defined as metallurgical chemistry, since he is working working at the border between chemistry and extractive metallurgy. Koen is the head of the SOLVOMET Group, the Laboratory of Metallurgical Chemistry @ KU Leuven (Belgium).
Koen Binnemans was born in Geel (Belgium) in 1970. He obtained his M.Sc. (1992) and Ph.D. (1996) degrees in Chemistry at the University of Leuven (KU Leuven). In the period 1999−2005, he was a postdoctoral fellow of the Research Foundation Flanders. He did postdoctoral work at the universities of Rennes (France) and Exeter (UK). From 2002 until 2005 he was (part-time) associate professor, from 2005 until 2010 professor, and since 2010 he is full professor of Chemistry at the University of Leuven. Koen is an inorganic chemist who is combining fundamental and applied research in a unique, double-sided interaction model. His main field of expertise could be defined as metallurgical chemistry, since he is working working at the border between chemistry and extractive metallurgy. Koen is the head of the SOLVOMET Group, the Laboratory of Metallurgical Chemistry @ KU Leuven (Belgium).
In 2017, he founded together with Peter Tom Jones the SOLVOMET Industrial Service Centre to support SOLVOMET’s industrial and RTD partners in the conceptual and practical development of more sustainable hydrometallurgical and solvometallurgical separation processes. Koen has published about 600 papers in international scientific journals. His work has been cited over 43,000 times and his h-index is 100 (Google Scholar). He is an elected Member of the Royal Flemish Academy of Belgium for Science and the Arts (KVAB). He was elected Chemistry Europe Fellow (Class 2016/2017) and he is Fellow of the Royal Society of Chemistry (UK).
Koen is married to Lieve Laureys and they have one son, Alexander.
Want to know more about SOLVOMET ISC and SIM²?
SOLVOMET ISC is KU Leuven’s Industrial Service Centre for Circular Hydrometallurgy. SOLVOMET ISC supports mining, metallurgical & recycling companies in the development of more sustainable (circular, low-energy input) hydrometallurgical processes, using state-of-the-art lab & mini-pilot scale experimental facilities. You can find out about SOLVOMET’s expertise, team and project types through these links:
- SOLVOMET ISC website: https://solvomet.eu/
- SOLVOMET ISC LinkedIn page: SOLVOMET ISC: | LinkedIn page SOLVOMET Industrial Service Centre
SIM² KU Leuven is the KU Leuven Institute for Sustainable Metals and Minerals (SIM² in short). SIM² is one of the official KU Leuven Institutes that were endorsed by the KU Leuven Academic Council. SIM² has more than 240 members, coming from a wide range of (interdisciplinary) research groups and departments at KU Leuven. SIM²’s missions is “to develop, organise & implement problem-driven, science-deep research & future-oriented education, contributing to the environmentally friendly production & recycling of metals, minerals & engineered materials, supporting (…) a climate-friendly, circular-economy”.
- SIM² website: https://kuleuven.sim2.be/
- Follow SIM² on LinkedIn: SIM2 KU Leuven: | LinkedIn page SIM² KU Leuven
_19832.png)


